|
Tania R.E –Esteban 1
School of Biology, Faculty of Biological science, University of Leeds, UK, LS2 9JT
The global threats facing keystone species is significantly impacting levels of biodiversity, due to the disproportionate effects keystones have on entire communities. They influence trophic interactions and provide ecosystem services of vital importance to the economic, social and cultural well-being of humans. It is therefore in our interest to establish the threats, the individuals most at risk, the potential cascading effects on ecosystems and how we are to manage them successfully in terms of reintroduction or mitigation. In this essay I review the major threats to a variety of different keystone species (at all trophic levels), examine how this influences levels of biodiversity and what effects they have on entire ecosystems. I also evaluate the current and potential management strategies that facilitate networks and allow them to be more resilient to future environmental change. Our knowledge of the concepts that underpin the fundamental basis of ecology can help us confront this as one of the greatest challenges in ecology.
Concept of Keystones
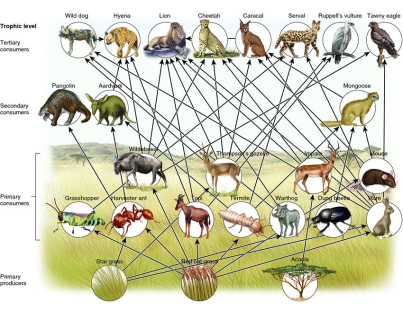
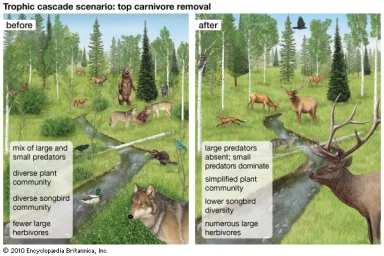
In different ecosystems, each specie plays a role within a community and can influence levels of biodiversity. However, the relative impact of each species can vary in terms of importance [27]. Such species that have disproportionate effects on ecosystems are known as keystone species [39]. According to network theory, keystones are intimately linked via ecological networks of highly connected and complex webs (Box 1, [9]). These include species at different trophic levels. Apex predators exert top-down effect on these levels, known as trophic cascades; whereby these strongly connected species indirectly influence community structure and ecosystem function [37]. The robustness of food webs to species removals varies, depending on the species and ecosystem type, where certain removals have greater impacts on ecosystem functioning and structure. Many apex predators are classed as keystone species because of the secondary extinction impacts of their removal on other species [7]. Predators directly impact upon herbivore numbers as well as indirectly through risk effects [34]. This then influences the relative abundance of producers- hence a ‘cascading effect.’
Equally, predators sustain levels of biodiversity via the suppression of other competitors (mesopredators) through competitive exclusion, and allow other species to co-exist [20]. Predatory release occurs when the apex predator is removed, increasing populations of the less competitive mesopredator. This then leads to a decline in its prey. Predator-prey dynamics as well as competition between intra and interspecific species also influence the structure of the food webs [1, 27]. The length of food webs can also greatly influence the direction of the cascade according to the exploitation ecosystem hypothesis [11]. These natural processes can be perturbed by threats to apex predators- whereby the removal of such keystone species leads to the concept of trophic downgrading [10]. As well as this, there is an alternate stable; where ecosystems are disturbed to such an extent that the cascade shifts from its prior state to another- when a tipping point is reached [10].
Box 1- Network theory
The fragile nature of ecosystems has been explored by Sole and Montoya [36], on the basis that if the nodes that connect individuals are randomly removed in a network, it remains stable. However, when highly connected individual are removed, this results in cascading effects and interference throughout the rest of the network. These keystone individuals form the framework and structure of the network. In real ecological networks, strong evidence for the removal of predators are known to not only directly impact its prey, but also have indirect effects via top-down forcing. Ecosystems processes such as primary production, nitrogen cycling and the establishment of invasive species are also affected (Figure 3 [10]).
Keystones – threats to a complex web of interactions
Habitat destruction
There have been major declines in biodiversity within recent decades, in what has been described as the 6th mass extinction event [27]. The threats facing keystones and the ecosystem services they provide are predominantly anthropogenic [19], and habitat loss is arguably one of the greatest [15]. For example, the Yellow and Black-Casqued Hornbills are both in decline, which has been correlated with deforestation in Nigeria as well as forest sections along the Ivory Coast [28]. This is problematic in that the genera Ceratogyma are key seed dispersers of fruiting trees, and play an important role in maintaining the heterogeneity of forests and species diversity via gene flow [28]. Because of the large spatial distribution of their territories [45], up to 22% of lowland tropical rainforest species are dispersed by the 3 hornbill species within this genera [23]. Cultural ecosystem services include traditional ceremony wear as well as other benefits to the keystone tree species, Ficus, which in turn provides economic services to local tribes’ people. It is also an important food source for other species within the ecosystem [23].
Urbanization
Other threats to keystones include the urbanization of many habitats. Increased contact between humans and species drive them to exhibit behavioural plasticity and alter their behaviour [33]. A majority of studies indicate that increases in urban environments decreases species richness [35], due to disturbances in breeding patterns, anti-predator behaviour, fitness, selection of habitat and overall population size. This has cascading effects along trophic levels [2]. The black-tailed prairie dog, a keystone specie, contributes to the health of steppe habitats by mixing the soil. This increases plant productivity and landscape heterogeneity as well as providing coyotes with a food source [3]. Their overall numbers have decreased as a result of increased urbanization. However, in contrary to the risk-disturbance hypothesis, where increases in anti-predatory behaviours (such as vigilance) are seen, some populations exhibiting behavioural plasticity have reduced their vigilance due to habituation [12]. This has negatively impacted the vegetation due to increased foraging time, which has ‘rebounding’ effects back up the trophic cascade, on other herbivores and predators [33].
Climate change
Complex plant-pollinator webs are also disturbed by habitat destruction due to their sensitivity to perturbation [29]. This is the case with the keystone plant mutualist, Heliconia tortuosa,
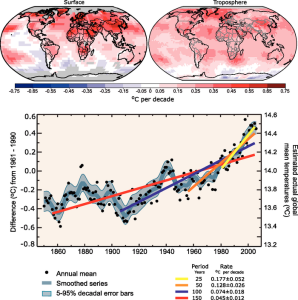
| Figure 1. The warming trend set to continue: (Left) Projected increases in temperature by 2081-2100 if mitigation and use of renewable resources is adopted. (Right) These are the predictions if the business as usual strategy continues (source: IPCC, Fifth assessment, 2014). |
which supports a variety of hummingbird species, and is considered a central node in this web interaction [17]. Recent work has provided evidence for the fragmentation hypothesis, where forest composition is fundamental to the reproduction of H. tortuosa. Thus, the reduction in heterogeneous landscapes due to deforestation is thought to alter both plant distribution and pollinator behaviour, leading to declines in both populations [29]. Equally, other systems have also shown that deforestation alters pollinator behaviour. Phaethornis hummingbirds will take longer flight paths to avoid deforest patches and agricultural landscapes, decreasing pollinator efficiency. This affects the survival of plant species dependent on this mutually beneficial ecological interaction- and has led to regional declines in biodiversity [16].
Climate change also poses a major threat to the biodiversity of keystone pollinators (such as bats, bees and birds [19]). One third of the world’s crop production is met by the ecosystem services provided by insect pollinators [30], with agricultural pollinator services estimate to be worth £120 billion per acre, annually [40]. Phenological shifts are also increasingly being observed, with the impact evident in both pollinator and plant keystone species [4]. In Japan, seed production in Corydalis ambigua and Gagea lutea decreased due to the warmer temperatures causing them to bloom earlier, resulting in phenological mismatching with its key pollinator, Apis Mellifera. Consequently, this reduced pollination efficiency and success [31]. Climate change has also altered bee distributions and caused phenological shifts in their flight period. Predictions suggest that the spatial shifts in bee movements will be faster than that of its food resource, also causing phenological mismatching and decrease wildflower pollination [4]. Therefore, climate change poses not only a threat to the keystone plant-pollinators, but to other communities dependent on wildflower meadow species [31]. This highlights the fragility of these mutualistic interactions as key nodes in an ecosystem, due to their varying response to temperature change.
A major issue with climate change is predicting the influence it will have on biological communities in the future [13]. The polar bear (Ursus maritimus) is an apex predator in the Arctic ecosystem which is very sensitive to changes in sea ice cover, where it hunts, migrates and reproduces [25]. The rate of temperature increase in the Arctic and northern regions have doubled in recent years, reducing sea ice cover [24]. In particular, over the past 30 years, the Western Hudson Bay has seen earlier ice break up as well as reduced snow fall (Figure 2). The impact on ringed seals (a keystone specie) with longer ice-free summers has subsequently lead to changes in polar bear behaviour [25]. The continuity of this pattern threatens seal pup survival as they are forced to swim for longer periods of time in open water, exposing them to predation [13]. Ringed seals provide polar bears with net wet weight calorific gains of 2.2-5.3 kcal/g [43], and the decreased recruitment of ringed seals has driven polar bears to target nesting birds, as they are unable to gain sufficient energy [25]. This warming trend is set to continue with possible increases in temperature of 5.0-6.4˚C by 2081-2100 (Figure 1, [24]).
Figure 2: Sea ice extent between 1979-2012 throughout the summer months. Evident loss seen annually. (Source: Iverson [25]).
Hunting and over-exploitation
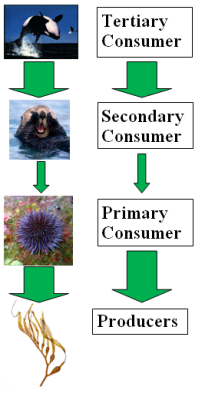
Hunting and over exploitation is also a prevalent threat to many keystones worldwide. The removal of a keystone predator is a major cause of secondary extinctions, demonstrating the strong influence of top-down effects on lower trophic levels [10]. This was seen with the expatriation of the Grey wolf in Yellowstone during 1935 (due to hunting), which increased populations of elk as a result of predatory release [39]. Increased levels of browsing on aspen, cotton and willow saplings in riparian river systems led to a more homogenous landscape and reduced diversity [34]. The wolf played a vital ecosystem role by maintaining diversity as well as healthy numbers of mesopredator populations. Classic studies of the consequences of predator removal are also illustrated with sea otters [10]. Enhydra lutris was nearly hunted to extinction by Russian fur traders at the beginning of the 20th century, resulting in the predatory release of sea urchins, which reduced Kelp forests by intensively over-browsing [42]. However, with the return of the otter during the 1970’s to certain areas, the recovery of the kelp forests was observed due to the effects of top-down control on urchins. The kelp populations in regions where otters were unable to recolonize did not recover [11], demonstrating how the impact of hunting can alter and result in the simplification of food webs.
The general pattern of global declines in apex predators is a cause for concern, due their strong connectedness in ecosystems and influence in altering the stability of food webs [19]. This is less well studied in marine ecosystems [20]. For example, sharks are apex predators in marine ecosystems [22], and are threatened by hunting. Demand for shark fin during the 90’s increased mortality rates by 80%. Many debate the function of sharks as keystone predators [8], however more recent studies suggest that although not all shark species can be described as keystones, some are key in structuring some ecosystems [44]. Indeed, strong arguments made by Estes et al., [10] concluded that the top-down effects exerted by apex predators are equally as influential as bottom-up effects. In Western Australia, Tiger sharks are considered a keystone apex predator; as mesopredator diversity (dolphins) and herbivores (dugongs) abundance are indirectly affected by the “seascape of risk,” as well as by direct predation [20]. 15 years of data collection in Shark Bay has supported the idea that the non-predatory effects of top apex consumers (predator keystones), play a pivotal role in influencing ecosystems [10]. Thus shark declines are affecting mesopredator numbers and behaviour, with unknown consequences on the rest of the aquatic communities [44].
Clearly, hunting apex predators can have detrimental, aggregating effects on lower trophic levels, both directly (via predation) or indirectly (through the landscape/seascape of fear concept). Equally, the idea that keystone’s play a role within and across communities [27], was seen with the decline of sea otters in the Aleutian archipelago populations due to increased predation by Orca [42]. The overexploitation of fish stocks in these waters in turn reduced populations of pinniped that fed on them [11]. This altered the orcas behaviour which began targeting otters as an alternative food source. Kelp forests once again declined as urchins were free of predation. This is known as the exploitation ecosystems hypothesis, where the food-chain length determines the level of influence and control top-down or bottom-up systems have in the primary productivity of ecosystems [10, 11].
Future: Management, mitigation and reintroduction
Problems
In terms of keystone species, the challenges of managing and mitigating their decline arise due to the complexity and interconnectedness between the many species they affect within ecological communities [5]. For example, managing the declines of seed dispersers is hard due to the large spatial ranges of their territories and difficulty in quantifying dispersal rates [23]. The extent to which urban-adapted keystones will affect the rest of the community depends on their ability exhibit behavioural plasticity and adapt, which varies between species and landscape scale [35].
The threat of climate change is also difficult to predict, thus is hard to prevent plant-pollinator loss in the future as phenological shifts continue at different rates [4]. Similarly, the uncertainty surrounding model projections of sea ice loss threatens the future survival of many Arctic species [25]. In aquatic systems, the lack of protection out of marine conservation areas and the extensive movements of keystone predators such as sharks, creates problems in managing and mitigating their decline [21, 22].
Equally, the overall concept of what constitutes a keystone species provides difficulties in management [7]. The definition can be broadly used to describe a spectrum of keystone types, which is confusing and problematic for conservation policy [7]. With an increasing number of species being given ‘keystone status,’ the lack of consistency in defining them more scientifically is rapidly becoming a challenge in itself [27].
Identification of which trophic direction is most influential in affecting levels of biodiversity within communities is controversial [10]. Often, it is dependent upon the ecosystem as well as the keystone specie. Some argue that primary production controls ecosystems bottom-up [18]. Others believe predatory top-down control is more influential, and is currently gaining more support due to the mounting evidence that global predator declines have significant impacts on communities and ecosystem processes (Figure 3, [10]). However, even if top-down systems were recognisably more important in structuring ecosystems, it is hard to determine the most prevalent impact predators have in influencing interactions within food webs- directly (through predation/lethal) or indirectly (risk effects/non-lethal [34]).
Figure 3: Indirect impacts of apex predators on different ecosystem function and processes (Source: Estes et al [10]).
Indeed, this is the case with sharks, where the importance of risk effects might be underestimated [26]. In terrestrial ecosystems, the ‘landscape of fear’ as well as direct predation by wolves is being taken into account in studies of natural systems, (eg: Białowieża Forest, Poland) in order to consider the nonlethal effects on herbivores [32]. Proposals by Manning et al., [34] suggest that controlled experiments in the Scottish highlands would provide much needed data and viable evidence for their reintroduction. However, public opinion is often divided in terms of reintroductions [37], and the funding and costs of trial experimentation are deemed wasteful [32].
The nature of predicting ecosystem response with the removal of a keystone is also very difficult when taken from a stable environment- where prior knowledge of the response in unknown [36]. Only a few studies have examples of networks that are partially mapped [5], and the substantial lack of data on a range of ecosystems makes management difficult [36]. Much earlier research does not indicate the strength of each trophic link, thus it is difficult to compare to the current consistent and empirically accurate data. Even when disrupted, other factors such as intraspecific competition can alter the response, and may take many years for the effects to propagate in the ecosystem [10]. Current strategies by the US Endangered Species Act fail to incorporate this [41].
Possible solutions?
It is vital that scientists are able to quantitatively asses the relative contribution of each proposed keystone [36]. Only then can policy makers implement management strategies that target and focus on protecting species that have the most important functional role- rather than the rarest specie [27]. We must also therefore demonstrate their functional importance before policy-makers act on the impetus of the analogical term of a keystone [7]. Of equal importance is understanding the connectivity of networks as well as attempting to mitigate the threats facing keystone apex predators. The geographic spatial scales at which natural or previously manipulated experimental removal experiments varies enormously, thus must also be considered in future reintroductions and management plans [39].
As already established, complex ecological concepts are hard to manage as many factors feed into the function, stability and persistence of food webs; including biotic and abiotic factors [10]. It is clear how important abiotic interactions greatly influence ecosystems and community structure and function, and must be considered if we are to manage and mitigate the effects of current and future climate change [19]. Therefore, as the fifth assessment by the IPCC suggests, anthropogenic climate change policy should focus on mitigation by following resilience pathways and realising adaption measures towards a more sustainable future [24]. If we are to reduce the number of extinctions, policy must also address the source of the problem; fossil fuel consumption, to mitigate the severe effects it could have on vulnerable keystones and their habitat [24].
Other concepts such as network theory have helped explain how the systematic targeting of particular keystone individuals is far more destructive than random removal [36]. Therefore fisheries must implement this into their harvesting methods and reduce their impact on sharks by implementing annual moratoriums to prevent over harvesting. This will require international cooperation to account for the spatial movements of these keystone predators [44].
Conclusion
The threats facing keystone species may arguably have the greatest impact upon ecosystem function and stability globally [10]. Keystone predators in particular play an important role as their loss is a cause of many secondary extinctions [21]. The complexity of these networks cannot be undermined, and scientists must now be able to predict and further assess why certain keystone species are more robust or more at risk from collapsing early on than others. This will determine which species will have the greatest impact upon the stability and function of communities [46]. Additionally, completion of fully described networks will need to be of a multidisciplinary manner, in order to expand upon the current knowledge of these systems. Mitigating the threats as well as assessing the success of keystone reintroductions in influence levels of biodiversity is also key [44]. The reduction of harmful human activities is also necessary in order to prevent future extinctions and declines in biodiversity [24]. Ultimately, the solutions to the challenges facing keystones and ecosystem function are not simple. However, further knowledge of how these systems work and the implementation of efficacious management strategies will lead more efficient restoration and protection.
References
- Allesina, S, and Pascual, M. (2008) Network structure, predator-prey modules, and stability in large food webs. Theoretical Ecology 1, 55-64.
- Angeloni, L, et al. (2008) A reassessment of the interface between conservation and behaviour. Anim Behav 75, 731-737.
- Bangert, RK and Slobodchikoff, CN. (2000) The Gunnison’s prairie dog structures a high desert grassland landscape as a keystone engineer. Journal of Arid Environments 46, 357–369.
- Bartomeus, I, et al. (2011) Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proceedings of the National Academy of Sciences of the United States of America 108, 20645-20649.
- Berlow, EL, et al. (2009) Simple prediction of interaction strengths in complex food webs. Proceedings of the National Academy of Sciences of the United States of America 106, 187-191.
- Brose, U, et al. (2006) Allometric scaling enhances stability in complex food webs. Ecology Letters 9, 1228-1236.
- Cottee-Jones, et al. (2012) The keystone species concept: a critical appraisal. Frontiers of Biogeography 4, 117-127.
- Dulvy, NK, et al. (2008) You can swim but you can’t hide: the global status and conservation of oceanic pelagic sharks and rays. Aquatic Conservation-Marine and Freshwater Ecosystems 18, 459-482.
- Dunne, JA, et al. (2002) Food-web structure and network theory: The role of connectance and size. Proceedings of the National Academy of Sciences of the United States of America 99, 12917-12922.
- Estes, JA, et al. (2011) Trophic downgrading of planet earth. Science 333, 301–306.
- Estes, JA, et al. (1998) Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science 282, 473-476.
- Frid, A, and Dill, L. (2002) Human-caused disturbance stimuli as a form of predation risk. Conserv Ecol. 6, 31-54.
- Ferguson, SH, et al. (2005) Climate change and ringed seal (Phoca hispida) recruitment in western Hudson Bay. Marine Mammal Science 21, 121-135.
- Garibaldi, A. (2009) Moving from model to application: Cultural keystone species and reclamation in fort MCay, Alberta. Journal of Ethnobiology 29, 323-338.
- Gross, L. (2006) Predicting Species Abundance in the Face of Habitat Loss. PLoS Biol 4. (doi: 10.1371/journal.pbio.0040336)
- Hadley, AS, and Betts, MG. (2009) Tropical deforestation alters hummingbird movement patterns. Biology Letters, 5, 207-210.
- Hadley, AS, et al. (2014) Tropical forest fragmentation limits pollination of a keystone under story herb. Ecology 95, 2202-2212.
- Hairston, NG, et al. (1960) Community structure, population control, and competition. Am Nat 94, 421-424.
- Harley, CDG. (2011) Climate Change, Keystone Predation, and Biodiversity Loss. Science, 334, 1124-1127.
- Heithaus, MR, and Dill, LM. (2006) Does tiger shark predation risk influence foraging habitat use by bottlenose dolphins at multiple spatial scales? Oikos 114, 257–264. (doi:10.1111/J.2006.0030-1299.14443).
- Heithaus, MR, et al. (2008) Predicting ecological consequences of marine top predator declines. Trends in Ecology & Evolution 23, 202-210.
- Hinman, K. (1998) Ocean roulette: conserving swordfish, sharks and other threatened pelagic fish in longline-infested waters. Leesburg: Harvard Press.
- Holbrook, KM, and Smith, TB. (2000) Seed dispersal and movement patterns in two species of Ceratogymna hornbills in a West African tropical lowland forest. Oecologia 125, 249-257.
- IPCC, (Intergovernmental Panel on Climate Change). 2014. Summary for Policymakers in Impacts, Adaptation, and Vulnerability. Part A. United Kingdom and New York: Cambridge University Press.
- Iverson, SA, et al. (2014) Longer ice-free seasons increase the risk of nest depredation by polar bears for colonial breeding birds in the Canadian Arctic. Proceedings of the Royal Society B-Biological Sciences 281, 43-51.
- Johnson, SD, et al. (2009) Pollination and breeding systems of selected wildflowers in a southern African grassland community. South African Journal of Botany 75, 630-645.
- Jordán, F. (2009) Keystone species and food webs. Philosophical Transactions of the Royal Society B: Biological Sciences 364, 1733-1741, (doi:10.1098/rstb.2008.0335).
- Kemp, AC. (1995) The Hornbills. Oxford: Oxford University Press.
- Klein, AM, et al. (2007) Importance of Pollinators in Changing Landscapes for World Crops. Proceedings of the Royal Society 274, 303–313.
- Kremen, C, et al. (2007) Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecology Letters 10, 299-314.
- Kudo, G, et al. (2004) Does seed production of spring ephemerals decrease when spring comes early? Ecological Research 19, 255-259.
- Kuijper, DPJ. et al. (2014) What Cues Do Ungulates Use to Assess Predation Risk in Dense Temperate Forests? Plos One 9, 12-16.
- Magle, SB. and Angeloni, L M. (2011) Effects of urbanization on the behaviour of a keystone species. Behaviour 148, 31-54.
- Manning, AD, et al. (2009) Restoring landscapes of fear with wolves in the Scottish Highlands. Biological Conservation 142, 2314-2321.
- McKinney, ML. (2008) Effects of urbanization on species richness: A review of plants and animals. Urban Ecosystems 11, 161-176.
- Montoya, JM, et al. (2006) Ecological networks and their fragility. Nature 442, 259-264.
- Outland, K. (2010) Who’s afraid of the big bad wolf? The Yellowstone wolves controversy. [Online]. [Accessed 10 November 2014]. Available from: http://legacy.jyi.org/volumes/volume11/issue5/features/outland.html
- Platten, S. and Henfrey, T. (2009) The Cultural Keystone Concept: Insights from Ecological Anthropology. Human Ecology 37, 491-500.
- Ripple, WJ, and Breschetta, RL. (2004) Wolves, elk, willows, and trophic cascades in the upper Gallatin Range in Southwester Montana, USA. Forest Ecology and Management 200, 161–18.
- Schulp, CJE, et a (2014) Quantifying and mapping ecosystem services: Demand and supply of pollination in the European Union. Ecological Indicators 36, 131-141.
- Soulé, ME, et al. (2005) Strongly interacting species: conservation policy, management, and ethics. Bioscience 55, 168-176.
- Steneck, RS, et al. (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environmental Conservation, 29, 436-459.
- Stirling, I, and McEwan, EH. (1975) Calorific value of whole ringed seals (Phoca Hispida) in relation to polar bear (Ursus Maritimus) ecology and hunting and behaviour. Canadian Journal of Zoology-Revue Canadienne De Zoologie 53, 1021-1027.
- Techera, EJ, and Klein, N. (2007) Sharks: Conservation, Governance and Management. Earthscan, 155-175.
- Trail, PW. (2007) African hornbills: keystone species threatened by habitat loss, hunting and international trade. Ostrich 78, 609-613.
- Worm, B, and Myers, RA. (2003). Meta-analysis of cod-shrimp interactions reveals top-down control in oceanic food webs. Ecology 84, 162-173.